Science Mom's Guide to Water, Part 2
Water has the strongest surface tension of any non-metallic liquid. In this guide we explore just how strong surface tension is--and how it can change--through several hands-on activities.
Science Mom’’s Guide to Water, Part 2
Water has the strongest surface tension of any non-metalic fluid. So for most of the liquids we come in contact with, water is the surface tension champion. Whether you’re comparing it to oils and alcohols or hydrocarbons like gasoline and lighter fluid, water will stand above the crowd. Literally.
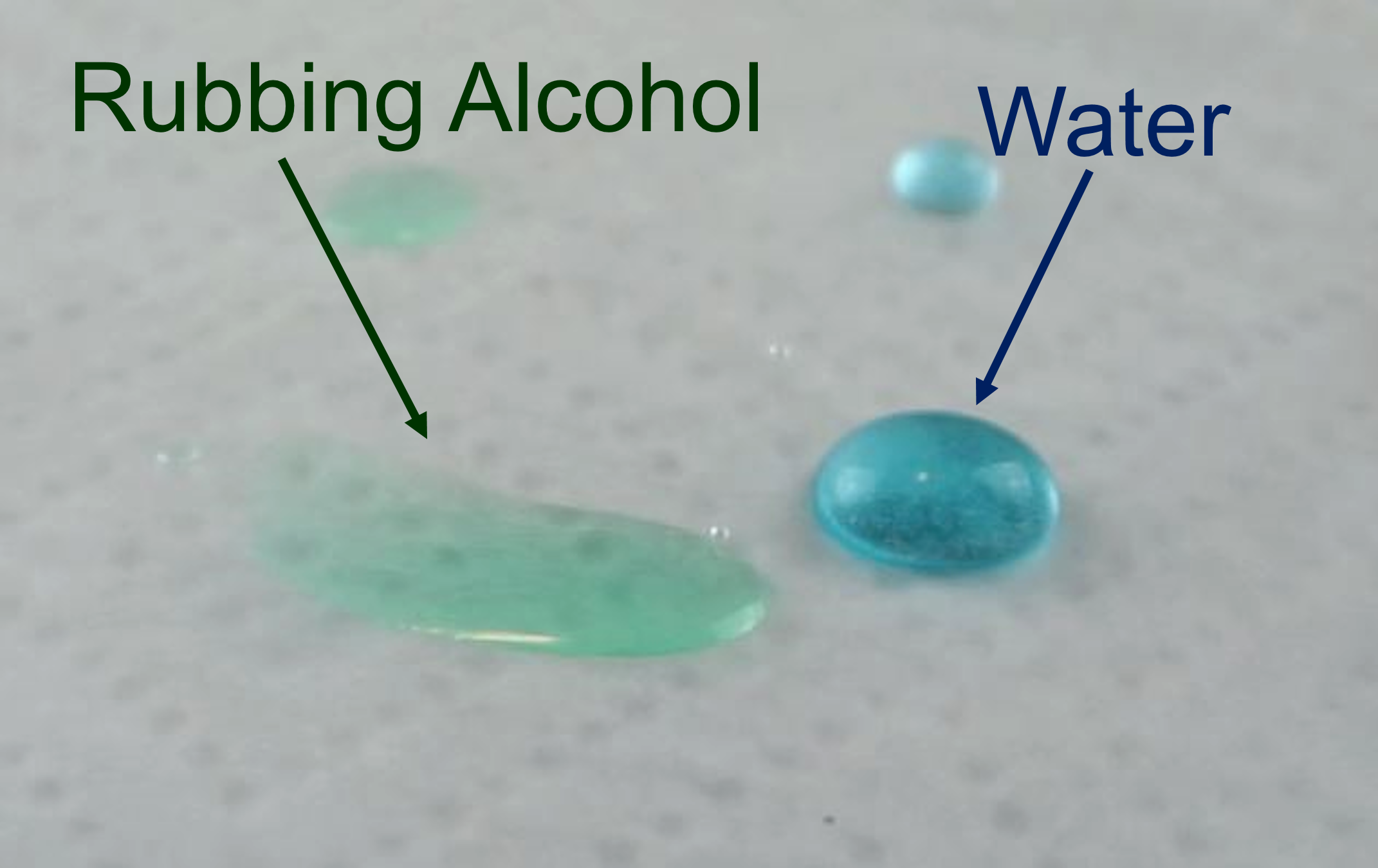
Water forms beautiful domes on waxed paper while other liquids remain rather flat. We can poke those drops or try to squash them with our fingers, and (so long as our fingers are clean), the drops will keep their dome-like shape. But if we dip a toothpick in soap and touch them? Then the surface tension of the water changes dramatically:

In the picture above, I touched every other drop with the soapy toothpick, and you can see how flat they got in comparison to their non-soapy neighbors! It doesn’t take very much soap to make a huge difference to the surface tension.
To learn more about why surface tension works and to see several fun investigations you can do with surface tension, watch Science Mom’s Guide to Water, Part 2:
You can preview the accompanying guide by scrolling through the pages in the viewer below. To print your own copy, just select your preferred size of paper: 8½ × 11, 11 × 17, A3 or A4. Don’t forget to print “Actual size.” Do not print “Fit to page” or the book won’t come out right. To fold the book, either print the two-page document double sided and follow the instructions on the back, or follow the instructions in this video: How to Fold A Book from One Piece of Paper.
And join us next time for Science Mom’s Guide to Water, Part 3 [coming February, 2017], where we investigate capillary action and adhesion.
Guide Preview

